Lab Activity 3: Heating and Cooling Curves of a Pure Substance
Objective:
To demonstrate the physical aspects of a phase change and to plot the cooling and heating curves of the change.
Discussion:
When a solid substance turns into a liquid, it is undergoing a phase change. A phase change is a physical change which does not involve the formation of anew substance. Rather, the substance undergoes a change in state or form. Heat energy is involved in a phase change, but the temperature remains constant during the period in which the phase changing.
In this investigation, you will use a moth deterrent, paradichlorobenzene, which has a low melting point. You will record the temperatures per unit time of the the melting and solidifying paradichlorobenzene. At the conclusion of the investigation, you will be expected to graph your results. If done accurately, your graph will emphasize the interesting aspects of the cooling and heating curves to a phase change.
Materials needed:
| Safety glasses | 15g of paradichlorobenzene | Bunsen burner |
| 250-ml beaker | watch with second hand | 2 thermometers |
| large test tube | wire gauze | ring stand |
| burette clamp | metal ring |
*See below for tips on performing this lab.
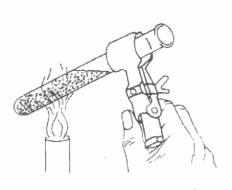
*Before you begin to record data for the cooling curve, you must melt the paradichlorobenzene. (See figure above.) Make sure you use a Burette clamp and not a test tube holder.
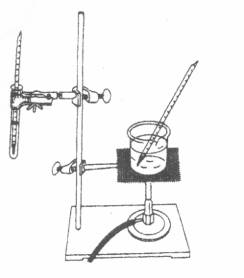
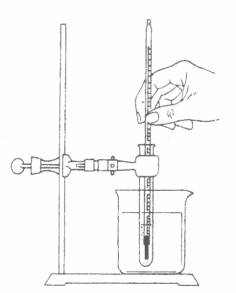
*When you are ready to begin the heating curve procedure, heat-up a water bath (Above-left figure) to 75 C first. Turn off the Bunsen burner and submerge the solidified paradichlorbenezene (within the test tube) in the water bath (above-right figure) so the level of the water is just above the level of the paradichlorbenezene. Immediately begin to record your measurements.
SFP Home | Science Home | Barron's Web Site | Lab Index | WebChem