Lab Activity 12: Relating Moles to Coefficients of A Chemical Reaction
Background:
The mole is defined as Avogadro's number (6.02
*10^23) of particles. These particles may be atoms, molecules, formula
units, ions, electrons, etc. The concept of the mole is very important,
especially when dealing with quantitative studies of chemical reactions.
When calculating quantities of solids or liquids, molar masses are used.
The molar mass of a substance is the mass, in grams, of 1 mole of particles
of that substance. When calculating quantities of gases, molar volumes
are used. The molar volume (22.4 L) is the volume occupied by 1 mole of
a gas at STP.
Chemical reactions are represented by balanced chemical equations. Proper
interpretation of an equation provides a great deal of information about
the reaction it represents and about the substances involved in the reaction.
For example, the coefficients in a balanced equation indicate the number
of moles of each substance. Thus, the ratio of moles of a substance to
moles of any other substance in the reaction can be determined at a glance.
In this experiment, iron fillings will be added to an aqueous solution
of copper (II) sulfate. A *single
replacement reaction will take place, the
products being iron (II) sulfate and copper metal. The balanced equation
for this reaction is: Fe(s) + CuSO4(aq) à FeSO4(aq) + Cu(s)
The quantities of iron and copper sulfate used as reactants will be such
that the copper sulfate will be in excess. Thus, the iron will be the
*limiting factor in determining the number
of moles (gram-atoms) of products that will be formed. As the equation
shows, the number of moles of copper produced should be equal to the number
of moles of iron reacted.
This experiment should aid in the understanding of balanced equations
and *single replacement reactions.
*Definitions of these terms are located below.
Purpose:
Find the ratio of moles of a reactant to moles of a product of a chemical
reaction. Relate this ratio to the coefficients of these substances in
the balanced equation for the reaction.
Materials:
| Balance | Beaker, 100-mL | Wire Gauze | Graduated Cylinder, 100-mL |
| Burner | Beaker, 250-mL | Safety goggles | Copper sulfate crystals (CuSO4) |
| Iron ring | Glass stirring Rod | Ring Stand | Iron filings (Fe) |
| Lab apron |
Safety:
Tie back long hair and secure loose clothing when working with an open flame. Always wear safety goggles and a lab apron or coat when working in the lab.
Procedures:
1. Find the mass of a clean, dry 100ml
beaker. Record this in your data table.
2. Measure out 8.0 grams of copper sulfate crystals (CuSO4) and add these
to the beaker.
3. Measure 5.0 ml of water in a graduated cylinder and add it to the crystals
in the beaker. While one lab partner continues with steps 4 and 5, the
other partner should carry out the instructions in step 6.
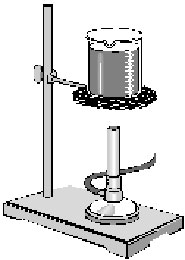
4. Set up the apparatus as shown in the figure 12-1. Keep the mixture
in the beaker to just below boiling. DO NOT ALLOW THE LIQUID TO BOIL.
5. Continue heating and stir mixture until the crystals are completely
dissolved. Turn off the gas and remove the burner.
6. Using the balance, measure precisely 2.24 grams of iron filings. (Remember:
do not place any reagent directly in the balance pan, use weighing paper.)
Record this mass in the data table.
7. Add the iron filing, small amounts at a time, to the hot copper sulfate
solution. Stir continuously. After all the iron has been added and the
mixture is stirred, allow the beaker to sit for 10 minutes while the reaction
proceeds. Record your observations in the data table.
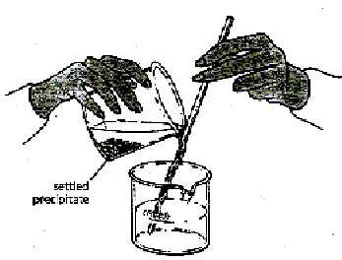
8. *Decant the liquid into a 250ml beaker
as show in figure 12-2 do not disturb the solid at the bottom of the beaker.
9. Add about 10mls of water to the solid in the 100ml beaker. Stir vigorously
in order to wash off the solid. Let the solid settle and *decant
the liquid. Repeat the washing.
10. Spread the solid out on the bottom of the beaker, label the beaker
with your period and group name, and place the beaker in an oven to dry.
Complete step 11 and the rest of this experiment at the beginning of the
next lab period.
11. Find the mass of the beaker and the dry copper metal. Record this
in your data table.
| Figures: | |
 |
 |
| Figure 12-1 |
Figure 12-2 |
Definitions of New Terms:
Decant
1 : To draw off (a liquid) without disturbing the sediment or the lower
liquid layers.
Limiting Reactant
1 : Substance that stoichiometrically limits the amount of product(s)
that can be formed.
Single Replacement Reactions (always
redox)
1 : An uncombined element replaces another element like it (similar charge
sign) from a compound.
a) metals will replace other metals (cations or positive charges replace
other positive charges)
b) nonmetals will replace nonmetals (anions or negative charges replace
other negative charges).
SFP Home | Science Home | Barron's Web Site | Lab Index | WebChem